Minerals
Back to the GEOL 102 Homepage
MineralsBackgroundMineral SamplesOlivineAugiteHornblendeMica Mineral GroupFeldspar mineral groupPlagioclase Feldspar:Orthoclase Feldspar:QuartzTable of Mineral Formulas and PropertiesElement substitutionMineral CleavageUses of MineralsMinerals as Gemstones/JewelryOther NotesConnecting Minerals to RocksSlidesOther ResourcesJust For FunMiscellaneous
Background
Let's have an introductory look at earth's structure and composition (elements and minerals) → slides
You can do a lot of geology with just a handful of minerals. Here we will look at eight minerals we will be interested in for the near future:
Olivine → the most common mineral in the upper mantle.
Augite → the most common of the pyroxene mineral class)
Hornblende → the most common version of amphibole mineral class)
Common mica minerals
Muscovite → a common mineral of the mica group)
Biotite → a common mineral of the mica group)
Common feldspar minerals
Plagioclase Feldspar → often referred to as plagioclase or even just plag
Orthoclase Feldspar → often referred to as potassium feldspar or K-feldspar
Quartz
For fun: may the quartz be with you
For the purposes of this class there is no great harm in using, for example, pyroxene and augite interchangeably. But note that pyroxene is the more general mineral class, while augite is a particular realization of pyroxene (more on this in the table shown further down the page).
Mineral Samples
Olivine
A personal favorite 😍

Augite
Augite is the most common example from the pyroxene mineral group. Here is a page about augite.

Hornblende
Hornblende is the most common example from the amphibole mineral group.
What is meant in the image below by the term cleavage?

Mica Mineral Group
The two most common examples are biotite (left) and muscovite (right). Here is a link to a page about micas.

Feldspar mineral group
The two most common examples are plagioclase feldspar and orthoclase feldspar. In fact, plagioclase feldspar is the most common mineral in earth's crust.
Plagioclase Feldspar:

Orthoclase Feldspar:

Quartz
You have likely seen quartz before. Here is a link to a page about quartz.

Table of Mineral Formulas and Properties
Fear not - we are not here to memorize the chemical formulas. But we do want to see trends with there compositions and other properties such as density, cleavage patterns, etc. Nature has been very clever in finding ways to balance charge.
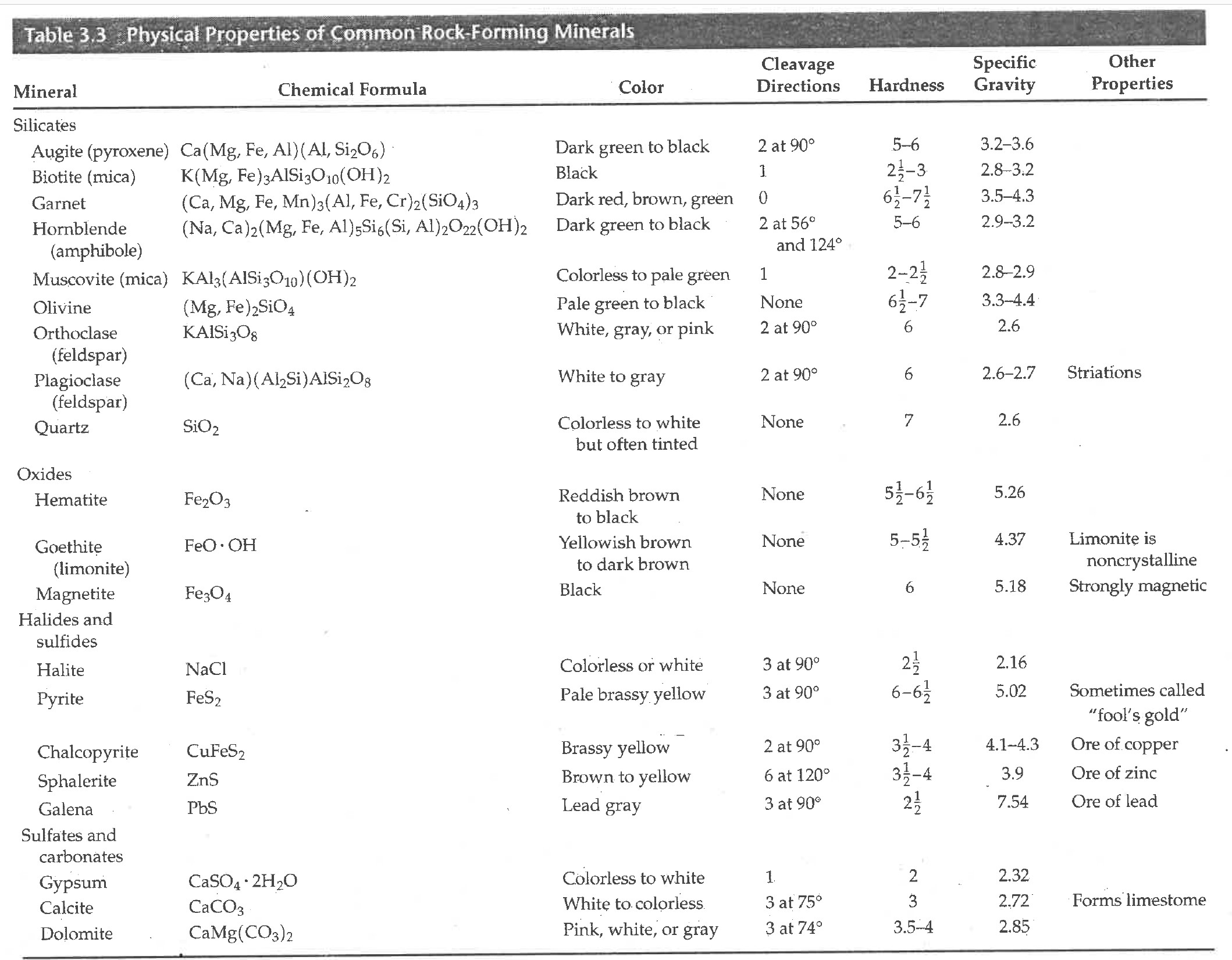
What we do want to know about these minerals are:
Substitutions and charge balance
Cleavage characteristics
Identify samples based on cleavage patterns and other characteristics (e.g., orthoclase is a pink color - although color can be misleading, mineral hardness, etc.).
Element substitution
Olivine has a fairly simple formula: (Fe,Mg)2SiO4.
This means that there are two 2+ cations of Mg, or Fe, or a combination. In other words, these two cations can substitute for one another in the formula.
Substitution is possible because (a) the ionic radius of the two cations are similar, and (b) the charges are the same.
Special Case Names:
You don't have to memorize these special cases (I sometimes mix them up myself), I just like olivine.
If both cations are Mg, i.e. Mg2SiO4 then the mineral is called Fosterite.
If both cations are Fe, i.e. Fe2SiO4 then the mineral is called Fayalite.
Here is a very beautiful sample of Fosterite: Geology Tweets on Twitter: "This is one of the best forsterite (Peridot) crystals we have ever seen from the famous Sapat Valley in Pakistan.
A short post on Titter: The uppermost mantle is green
Mineral Cleavage
Mineral cleavage is the tendency of a mineral to cleave (break) cleanly along planar surfaces (we think of parallel planes as being the same direction, so it is not counted twice).
Not all minerals have cleavage planes — the classic exmaple is quartz. Quartz has a very distince crystal habit (shape), but if you try to break it into smaller pieces it just shatters (conchoidal fracture, to be fancy). There is no plane on which it cleaves (breaks) cleanly. Quartz has zero planes of cleavage.
The mineral halite (salt!) is an example of a mineral with three planes of cleavage — and those planes intersect each other at 90 degree angles. If you break calcite, it just becomes a smaller version of itself:
Uses of Minerals
Many minerals are needed to make our phones and other devices (e.g. computers, solar panels, batteries, construction materials, etc.). Here is a link to a flyer from the U.S. Geological Survey about minerals in mobile devices. A lot of minerals are needed to make solar panels as well.
The necessity to identify and mine minerals is a significant geological, environmental, and political challenge.
I like to say that ...
... everything we have is either mined (geology) or grown (soil science).
These two categories (geology, soil science) even include things like extraction of groundwater, harvesting of trees (and related things), etc. Therefore, geology should be the most important subject on campus — the other being soil science, but I prefer geology personally 😉
We will spend more time on the uses of and societal need for minerals, and their geological setting, towards the end of the course when we look at energy and mineral resources.
Minerals as Gemstones/Jewelry
Minerals have long been used as gemstones, e.g. garnet.
An excellent natural sample of garnet:

Garnet cut and polished into a gem quality sample:

Another pretty mineral is celestine (also called celestite):

Other Notes
In your reading pay particular attention to:
The Silica Tetrahedra
Silica tetrahedra bonding styles: isolated, single chains, etc. and which minerals exhibit these
How the proportion of Silica to cations changes as you run through these variations
Note that the bonding styles affect mineral cleavage properties
Connecting Minerals to Rocks
Rocks are simply aggregates of minerals (but how those minerals form and come together in the different rock types varies). Here are a few slides
Slides
The set of slides mostly mimics what you will find in your chapter reading. I will likely use a part of it in class - especially the material on the silica tetrahedra. But do not rely on this as your primary learning resource — do the chapter reading first!!
Other Resources
Optional resources to peruse:
There is a really nice website that discusses mineral properties (streak, luster, HCl acid test, etc) here: https://omg.georockme.com/home
There are also some nice mineral sample images and info here and here.
It would be useful to spend some time on this site. For now we would focus on the eight minerals I've identified on this page, but as time goes on we will examine a few others such as calcite, talc, magnetite, garnet, and others.
Just For Fun
New minerals are discovered sometimes. Also, new properties of minerals are often discovered as well. Below are some links "just for fun" - you are not responsible for this material.
Historical - Detection of minerals in water (from the US Geological Survey): USGS on Twitter: Margaret D. Foster
Beautiful new emerald-green mineral described from Cornwall | Natural History Museum (nhm.ac.uk)
A specimen of smithsonite
The Wonderful World of Minerals - Science Connected Magazine
Some history of Raman spectrometry - light scattering and mineral identification. Named after Sir Chandrasekhara Venkata Raman.
The mineral scorodite - beautiful sample: "Plenty of beautiful sharp blue scorodite crystals on matrix from Clara Mine, Wolfach, Black Forest, Baden-Württemberg, Germany
Miscellaneous